Gaseous Components And Substances
from web site
For that reason, for the objective of nomenclature, an acid can be considered as a particle with one or more protons (H+) bound to an anion. For instance, HSO3-- is not an monatomic iridium acid molecule; it is an anion because it lugs a-- 1 cost. Despite the fact that it reveals acidic properties, it is called like a polyatomic anion. Instead, it must be named as an ionic compound because it includes a Na+ cation and also an HSO3-- anion. Therefore, it is called sodium bisulfite or salt hydrogen sulfite. To call the covalent compounds, call the electropositive element initially.
What ion means?
Ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Positively charged ions are called cations; negatively charged ions, anions.
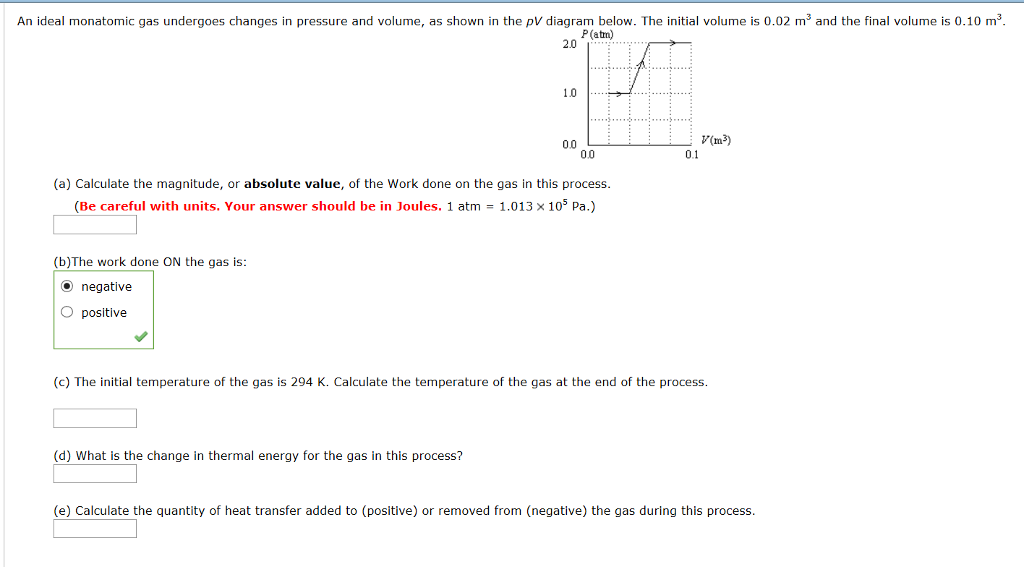
Extremely comparable residential or commercial properties, yet when you look at their whole atomic framework and also examine their latticework as well as subatomic properties, they are really different elements. When a heat Q is contributed to a monatomic perfect gas at constant stress, the gas does a work W.
Substances That Exist As Gases.
Both cyclohexene as well as cyclobutane are essentially nonpolar molecules, but cyclobutane has a substantially reduced molecular mass than cyclohexene, which once more has more than 4 carbon atoms. We as a result forecast that cyclobutane is most likely a gas at area temperature as well as stress, while cyclohexene is a liquid. Actually, with a boiling point of only 12 ° C, compared to 83 ° C for cyclohexene, cyclobutane is undoubtedly a gas at area temperature and also pressure.
Are all elements Monatomic?
Some elements are monatomic, meaning they are made of a single (mon-) atom (-atomic) in their molecular form. Helium (He, see Fig. Another form of oxygen, ozone (O3), has three atoms, and sulfur (S8) has eight atoms. All elemental molecules are made of atoms of a single element.

The tourist attraction in between adversely charged electrons and positively charged protons in an atom give the atom its structure. The strong pressure holds neutrons and also protons together in the nucleus. This force got its name because it is strong sufficient to get over the force of the favorably charged protons pushing back each various other. The number of electrons and protons in an atom determines its chemical properties. Chemical residential properties consist of the particular manner ins which atoms as well as molecules react and also the power that they release or utilize in these responses.
Re: Straight Molecule Turning Vs Nonlinear Particle Turning.
After that, call the a lot more electronegative element as if the a lot more electronegative element is an easy anion (finishing with-- ide). How does one understand which element is the electropositive component? If one follows this rule, then, SO2 would certainly be called sulfur oxide, and also Carbon Monoxide would be called carbon oxide. Very often, two nonmetals can incorporate to develop more than one substance.
- 3B, in which it can be seen that the characteristics of the monatomic ions in the fluid phase are spatially as well as temporally heterogeneous in their variation ranges, directions, and also times.
- A lot of the elements and also compounds we have actually run into until now are typically found as gases; a few of the a lot more common ones are listed in Table 10.2 "Some Typical Compounds That Are Gases at 25 ° C as well as 1.0 atm machine".
- As an instance, we take the trajectory of ion C in Fig.
- In addition, much of the easy covalent oxides of the nonmetals are gases, such as Carbon Monoxide, CARBON DIOXIDE, NO, NO2, SO2, SO3, as well as ClO2.
- Lastly, the majority of the generally made use of refrigerants, such as the chlorofluorocarbons and the hydrochlorofluorocarbons, which were discussed in Phase 3 "Chain Reaction", are gases.
Various other elements consist of 2 or more atoms in their molecular type (Fig. 2.8). An additional form of oxygen, ozone, has 3 atoms, as well as sulfur has eight atoms. All important molecules are constructed from atoms of a single component.
2, CO) easily get in deep spaces in the C60 latticework, specifically under a little overpressure, yet at low temperatures diffusion ends up being really slow. Noble gases intercalate without bonding but customize the mechanical homes and also the phase representations of fullerites, and the gas is released on pumping at heat. Hydrogen responds with fullerenes at high temperatures and also pressures creating substances which are unsteady on home heating at low stress.
Which Of The Following Is A Monatomic Cation? (a) Br (b) Nh_4 ^+. (c) So_4 ^ 2.
Some elements are monatomic, indicating they are made of a solitary (mon-) atom (- atomic) in their molecular type. Helium (He, see Fig. 2.8) is an example of a monatomic component. Hydrogen, oxygen, and chlorine molecules, as an example, each contains 2 atoms. The residential or commercial properties of some groups are so unique or crucial that the groups are described by unique names. The last group, Group 18, includes helium, neon, argon, krypton, xenon, as well as radon. The components in this group are called the noble gases.
