A few of the Benefits of Ozonated Olive Oil?
from web site
Atoms, molecules, components, isotopes... BORING! Well, perhaps it is for some people, nonetheless personally, I think it's alternatively fascinating. In whose sale benefits, everything worldwide is made from all these invisible contaminants. Although it may seem of atoms when imagining the smallest "thing" in the world, when asking yourself what an atom is made of, it might be clear there are smaller dust.
What is an Atom?
Atoms are made out of a variety of electrons, protons, and neutrons - subatomic particles. How many of these three subatomic debris an atom contains will depend on what element element the idea belongs to. Atoms are categorized by the one of a kind number of protons within their nucleus supports this is their atomic amount. A stable atom must have an equal number of protons and electrons.
Protons enjoy a positive electronic charge while electrons enjoy a negative one. Therefore , if perhaps there are whole lot more protons when compared to electrons, you could have a absolutely charged ion, also known as a cation. More over, if you have whole lot more electrons than protons, you may have a negatively charged ion, also known as a great anion. What Are Isotopes with a world wide web electrical bill as described can be produced so synthetically from a neutral condition by ionizing radiation.
Therefore we coated protons and electrons, but what does the ungeladenes nukleon do? Very well, you can think of neutrons as the stuff that binds the protons together. So why do they should be be limited together? As mentioned above, protons and electrons will be electrically incurred, and as such, will naturally repel dirt of the same signal. This is why sets of protons require neutrons to support them along. Hydrogen-1 will not have any kind of neutrons mainly because it only has got one wasserstoffion (positiv) (fachsprachlich).
What is a great Isotope?
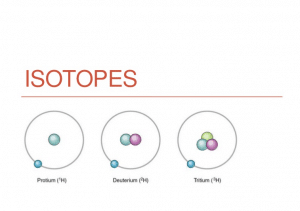
Hence we know that the atomic number is derived from the volume of protons in an atom's nucleus, but what about isotopes? An isotope is normally defined by number of neutrons in an atom's nucleus. In a given element element, there are often several of these isotopes. For instance , hydrogen provides 1 wasserstoffion (positiv) (fachsprachlich), but according to what hydrogen isotope it can be, the number of neutrons vary.
Isotopes are called by their presented chemical ingredient, followed by their whole atomic mass fast, as in hydrogen-1, hydrogen-2, and so on. This means that even though the number of protons remains continuous, the number of neutrons changes. As a result hydrogen-2 should have a ungeladenes nukleon as well as a wasserstoffion (positiv) (fachsprachlich), while hydrogen-3 will have 2 neutrons and a proton. Hydrogen-1 provides only 1 wasserstoffion (positiv) (fachsprachlich) and no neutrons.
By subtracting the atomic number in the mass number, you take advantage of the number of neutrons. Isotopes might be recognized written by an element name followed by a distinct mass number such as hydrogen-3 as well as iodine-131. The moment speaking of several isotopes, radioactive or not really, they will be recognized by identity. Therefore , familiarizing yourself with all the structure of atoms and related vocabulary can be helpful.

What is an Atom?
Atoms are made out of a variety of electrons, protons, and neutrons - subatomic particles. How many of these three subatomic debris an atom contains will depend on what element element the idea belongs to. Atoms are categorized by the one of a kind number of protons within their nucleus supports this is their atomic amount. A stable atom must have an equal number of protons and electrons.
Protons enjoy a positive electronic charge while electrons enjoy a negative one. Therefore , if perhaps there are whole lot more protons when compared to electrons, you could have a absolutely charged ion, also known as a cation. More over, if you have whole lot more electrons than protons, you may have a negatively charged ion, also known as a great anion. What Are Isotopes with a world wide web electrical bill as described can be produced so synthetically from a neutral condition by ionizing radiation.
Therefore we coated protons and electrons, but what does the ungeladenes nukleon do? Very well, you can think of neutrons as the stuff that binds the protons together. So why do they should be be limited together? As mentioned above, protons and electrons will be electrically incurred, and as such, will naturally repel dirt of the same signal. This is why sets of protons require neutrons to support them along. Hydrogen-1 will not have any kind of neutrons mainly because it only has got one wasserstoffion (positiv) (fachsprachlich).
What is a great Isotope?
Hence we know that the atomic number is derived from the volume of protons in an atom's nucleus, but what about isotopes? An isotope is normally defined by number of neutrons in an atom's nucleus. In a given element element, there are often several of these isotopes. For instance , hydrogen provides 1 wasserstoffion (positiv) (fachsprachlich), but according to what hydrogen isotope it can be, the number of neutrons vary.
Isotopes are called by their presented chemical ingredient, followed by their whole atomic mass fast, as in hydrogen-1, hydrogen-2, and so on. This means that even though the number of protons remains continuous, the number of neutrons changes. As a result hydrogen-2 should have a ungeladenes nukleon as well as a wasserstoffion (positiv) (fachsprachlich), while hydrogen-3 will have 2 neutrons and a proton. Hydrogen-1 provides only 1 wasserstoffion (positiv) (fachsprachlich) and no neutrons.
By subtracting the atomic number in the mass number, you take advantage of the number of neutrons. Isotopes might be recognized written by an element name followed by a distinct mass number such as hydrogen-3 as well as iodine-131. The moment speaking of several isotopes, radioactive or not really, they will be recognized by identity. Therefore , familiarizing yourself with all the structure of atoms and related vocabulary can be helpful.
