More About Protocol - How to Purify DNA from an Agarose Gel - Addgene
from web site

Supplementary information Materials and methods Soil Fundamentals Explained
QIAEX II Gel Extraction Set, For filtration of DNA fragments (40 bp to 50 kb) from gels and solutions, Package Content: Qiagen QIAEX II Gel Extraction Set, 500 Extractions, 20L Elution Volume, 5g/10L Binding Capacity, DNA Sample, Silica Technology, Handbook Processing, Tube Format, 40 bp to 50 kb Piece, For Filtration of DNA Fragments from Gels and Solutions, Perfect for Limitation Food Digestion, Identifying, Ligation, PCR, Consists Of 5 x 1m, L QIAEX II Suspension, Buffers, Efficient extraction of DNA from 40 bp to 50 kb.
No sodium iodide to disrupt subsequent responses. No shearing of big DNA fragments.
Top Guidelines Of Recommended Template Preparation
Filtration of DNA pieces with the QIAEX II system is based upon solubilization of agarose and selective adsorption of nucleic acids onto QIAEX II silica-gel particles in the existence of chaotropic salt. QIAEX II separates DNA from salts, agarose, polyacrylamide, dyes, proteins and nucleotides without phenol extraction or ethanol precipitation.
QIAEX II particles offer a slurry for gel extraction and ensure effective recovery without shearing, even for large DNA fragments. Enhanced buffers permit DNA healing without salt iodide, which is difficult to eliminate from DNA samples, and may impact subsequent responses. Official Info Here and binding buffer used with the QIAEX II system consists of an unique p, H sign.
Some Known Questions About A comparative study of extraction and purification methods for.
The colored color likewise enables easy visualization of any unsolubilized agarose in the binding mix, guaranteeing complete solubilization for maximum yield. p, H indication dye in the solubilization and binding buffer permits simple visual determination of optimal p, H for DNA adsorption (p, H 7. 5). An incorrect binding-mixture p, H can occur if the agarose gel electrophoresis buffer was often utilized or incorrectly prepared.
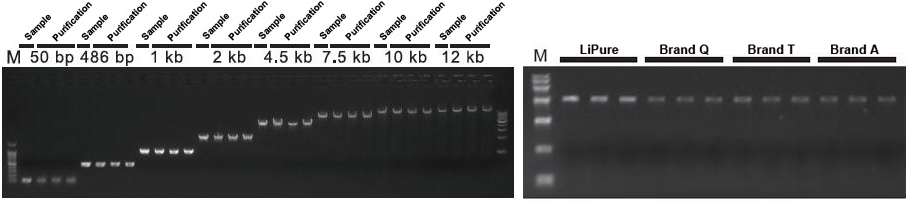
Anyone who has worked in a molecular biology laboratory understands that DNA gel extraction can be surprisingly challenging. Why is this? Is it due to the fact that of poor product yields, or maybe it's due to the fact that the gel extraction procedure uses harsh chemicals and conditions (e. g., chaotropic salts, ethidium bromide, ethanol, heat) that will damage or denature DNA and potentially reduce cloning success.
More About Zymoclean™ Gel DNA Recovery Kit w/ Zymo-Spin™ I

1. Cut the Gel Slice as Much as Possible, Get rid of all excess gel, including the gel in front of or behind your DNA band. The majority of individuals eliminated a square around the gel but don't believe to stand the excised piece up and cut the gel far from the front and back.
