What Does Sienna COVID-19 Antigen Rapid Test, 25/Box (SIENNA) Mean?
from web site

Evaluation of three immunochromatographic tests for rapid - An Overview

With this in mind, negative outcomes from an antigen test might need to be verified with a PCR test prior to making treatment choices or to prevent the possible spread of the infection due to an incorrect negative." Need More Info? -new code is intended for use as the industry requirement for accurate reporting and tracking of antigen tests utilizing immunofluorescent or immunochromatographic strategy for the detection of biomolecules produced by the SAR-Co, V-2 infection.

g., enzyme immunoassay [EIA], enzyme-linked immunosorbent assay [ELISA], immunochemiluminometric assay [IMCA] qualitative or semiquantitative, multiple-step technique; serious acute breathing syndrome coronavirus (e. g., SARS-Co, V, SARS-Co, V-2 [COVID-19]. Development, evaluation and approval of the brand-new CPT codes involved broad input from practicing physicians, industrial labs and other professionals. COVID-19 CPT coding assistance, consisting of brief and medium descriptors, is readily available from the AMA.
Additionally, the JAMA Network has an extensive overview of the coronavirusincluding public health, infection control and prevention recommendationsavailable on its JN Learning website. CPT Copyright 2020 American Medical Association. All rights booked. AMA and CPT are signed up trademarks of the American Medical Association.
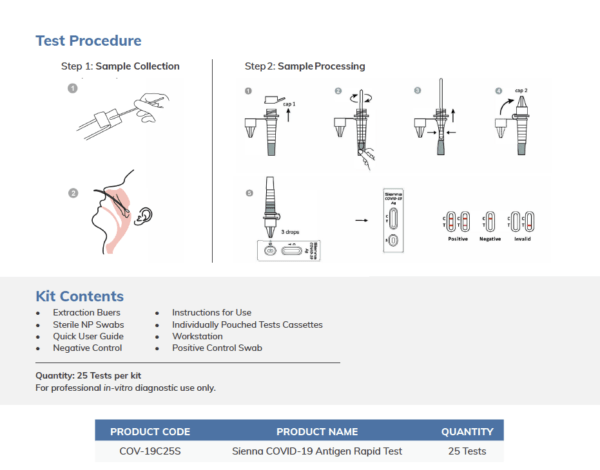
The Sienna COVID-19 Antigen Quick Test Cassette (Nasopharyngeal Swab) is a qualitative, lateral circulation immunoassay for the detection of the N protein of SARS-Co, V-2 in nasopharyngeal swab. In this test, antibody specific to the N protein of SARS-Co, V-2 is individually coated on the test line areas of the test cassette.
The Best Guide To New CPT codes released for COVID-19 testing, including
The mixture moves up the membrane to react with the antibody to N protein of SARS-Co, V-2 on the membrane and produce one colored line in the test regions. The existence of this colored line of the test areas shows a positive result. To function as a procedural control, a colored line will always appear in the control region if the test has carried out appropriate.
